Stable Triphenylamine Radical Cation
 New alternative to “magic blue” — a standard oxidant in organic chemistry — has been prepared and its properties were rationalized computationally.
New alternative to “magic blue” — a standard oxidant in organic chemistry — has been prepared and its properties were rationalized computationally.
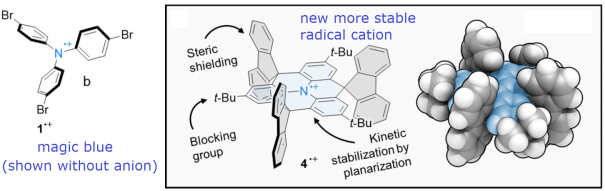
Magic blue is a salt of the radical cation of triphenylamine derivative and is commonly used as an oxidant in organic synthesis. Its use however is complicated by its slow decomposition into so-called “blues brothers”. The group in Germany led by Milan Kivala has prepared and experimentally characterized a more stable spiro-compound alternative also based on triphenylamine core.
The stability of the new radical cation has been ensured by careful design of the compound: triphenylamine has been planarized to improve delocalization of the radical over the π-system, bulky blocking groups were deployed in para-position to prevent dimerization, and large fluorenyl flanks were used orthogonally to the plane to additionally sterically shield the triphenylamine core.
The new radical cation has very clear absorption feature in the visible region making it potentially useful as a redox indicator. In addition, its preparation is rather simple and inexpensive.
DFT calculations confirmed that positive charge is mostly localized at the nitrogen center, which is well protected by fluorenyl groups.

We also performed analysis of the origin of the absorption bands with TD-DFT and found that the lowest energy bright states are mostly localized on the triphenylamine core rather than on fluorenyl flanks. This, in addition to the observation that the spin density is localized on shielded core, contributes to the remarkable stability of the radical cation.
This research was highlighted in Synfacts.
The work was published as an open-access article (CC BY-NC-ND 4.0). Raw computational data including geometries in xyz format in individual files can be downloaded from figshare.
- Tobias A. Schaub, Theresa Mekelburg, Pavlo O. Dral, Matthias Miehlich, Frank Hampel, Karsten Meyer, Milan Kivala*, A Spherically Shielded Triphenylamine and Its Persistent Radical Cation. Chem. Eur. J. 2020, 26, 3264–3269. DOI: 10.1002/chem.202000355.
0 Comments on “Stable Triphenylamine Radical Cation”