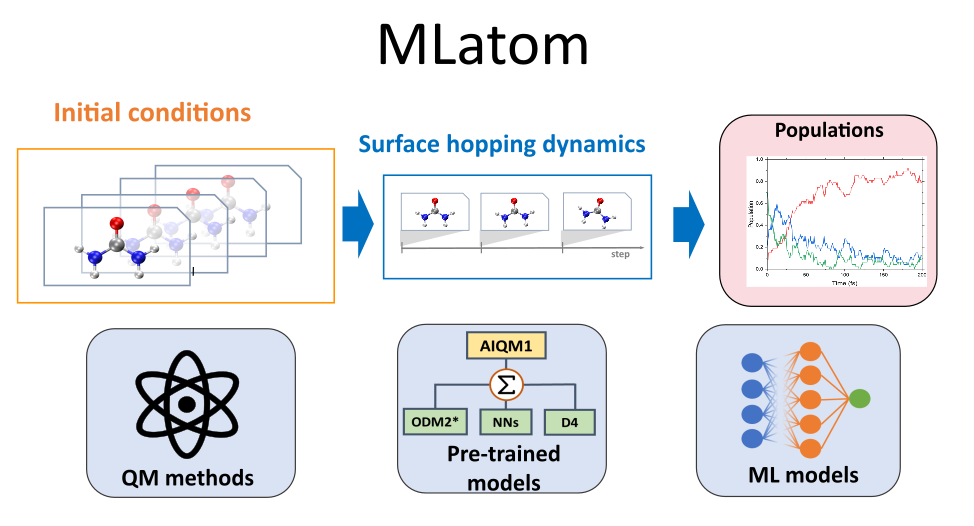
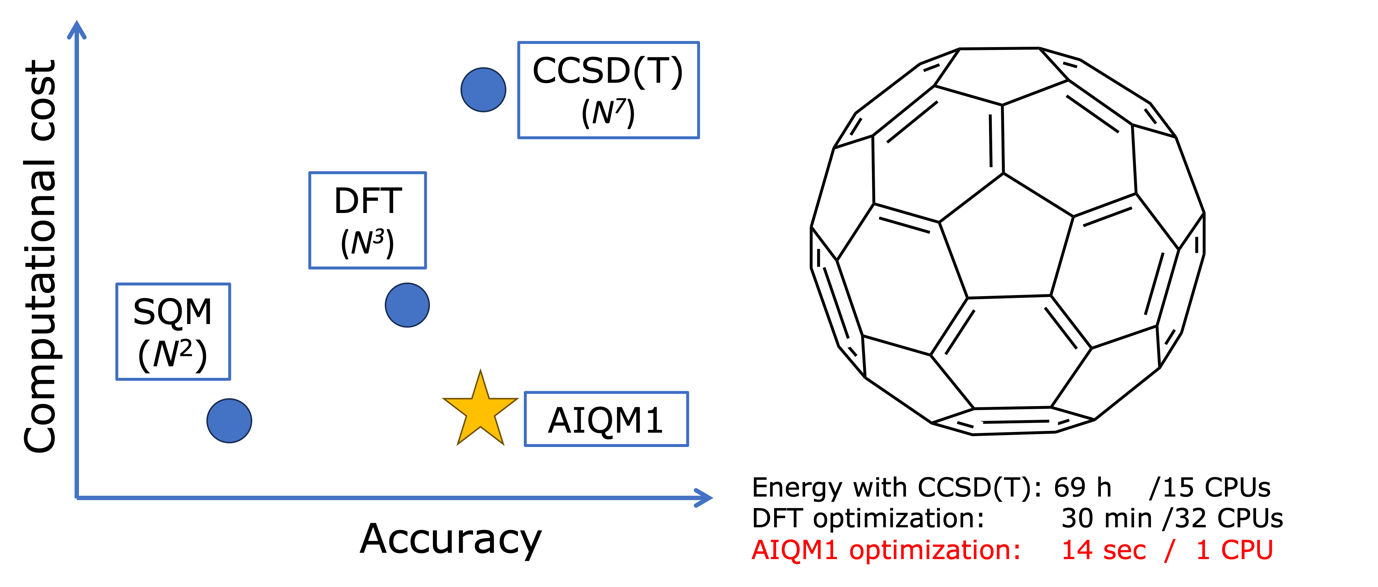
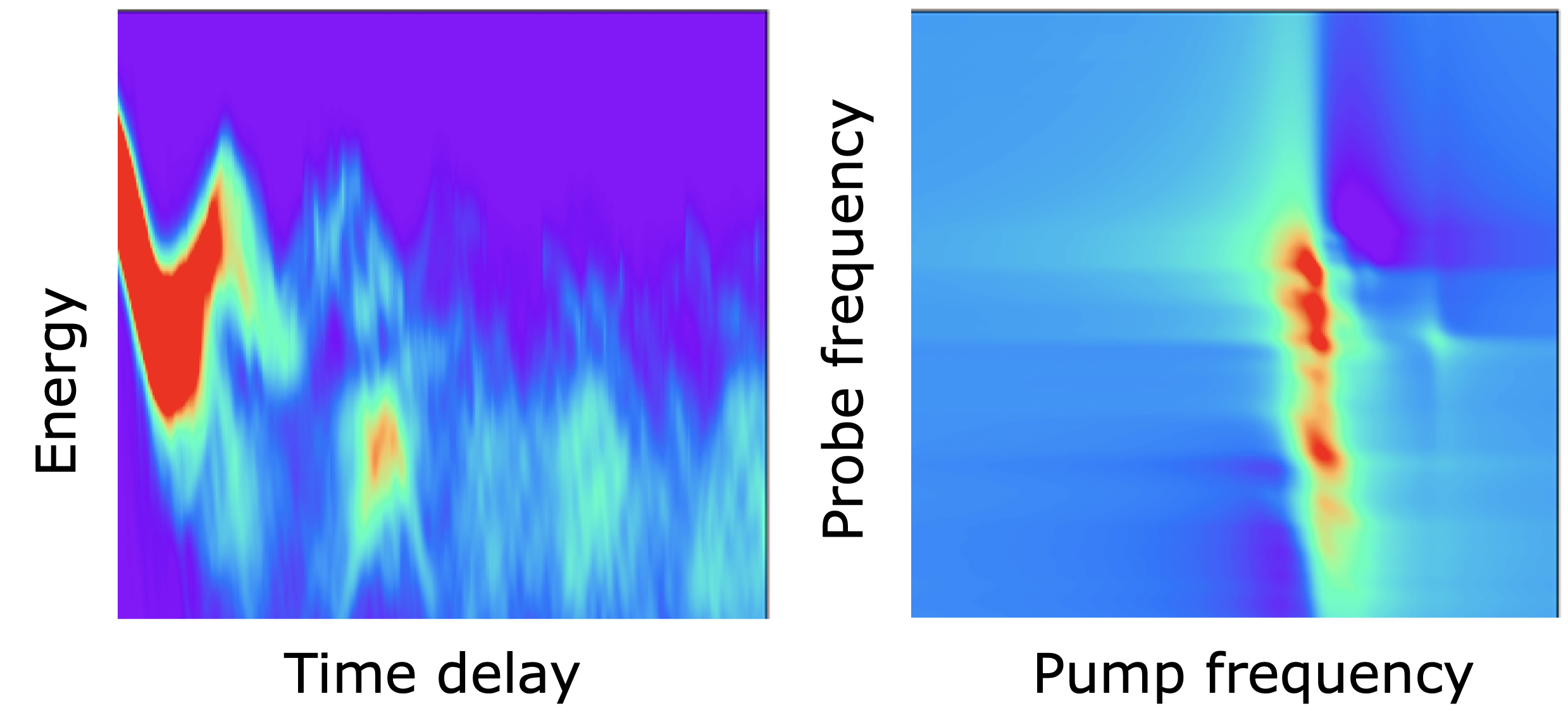
JCTC: Surface hopping dynamics with QM and ML methods
XACS team in collaboration with Mario Barbatti and groups in Warsaw University and Zhejiang lab has recently published a paper in JCTC about the versatile Python implementation of surface-hopping dynamics. This implementation is based on a powerful MLatom ecosystem for …
JCTC: Surface hopping dynamics with QM and ML methods Read more »