Electronic Structure Calculations
Nanoelectronics
We apply ab initio, DFT and semi-empirical methods to calculate different physicochemical and first of all electronic properties of compounds that are already used or are prospective materials for molecular (nano)electronics.
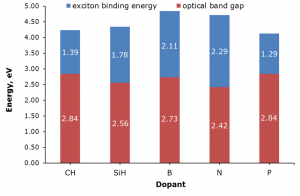
We have modeled the influence of internal doping of polycyclic aromatic hydrocarbon (PAH) as nanographene model on its electronic properties.[4] In this study we have also used methods (semiempirical UNO–CI) developed in our lab.[2]
We have explained the work of ambipolar transistor built from carbon peapod applying our own techniques (unrestricted EAL and IEL).[6] In addition electronic properties of the unusual endofullerene NH4+@C60 were studied.[5] We also carried out the theoretical investigation of the selective functionalization of oxadiamondoids that determine their orientation in devices and thus performance of such devices.[1]
In addition, we provided insights into bonding in coordination polymers, some of which are promissing materials for molecular electronics because of their redox properties.[7]
Publications
7. Nico Fritsch, Christian R. Wick, Thomas Waidmann, Pavlo O. Dral, Johannes Tucher, Frank W. Heinemann, Tatyana E. Shubina, Timothy Clark, Nicolai Burzlaff, Multiply Bonded Metal(II) Acetate (Rhodium, Ruthenium, and Molybdenum) Complexes with the trans-1,2-Bis(N-methylimidazol-2-yl)ethylene Ligand. Inorg. Chem., ASAP. DOI: 10.1021/ic501435a.
6. Pavlo O. Dral, The Unrestricted Local Properties: Application in Nanoelectronics and for Predicting Radicals Reactivity. J. Mol. Model. 2014, 20, 2134. DOI: 10.1007/s00894-014-2134-78.
5. Pavlo O. Dral, Theoretical study of electronic properties of carbon allotropes. [online] Friedrich-Alexander-Universität Erlangen-Nürnberg, Dissertation (Dr. rer. nat.), 2013, http://opus4.kobv.de/opus4-fau/frontdoor/index/index/docId/3763. [cited 2 February 2014]
4. Pavlo O. Dral, Milan Kivala, Timothy Clark, Doped Polycyclic Aromatic Hydrocarbons as Building Blocks for Nanoelectronics: A Theoretical Study. J. Org. Chem. 2013, 78, 1894–1902. DOI: 10.1021/jo3018395.
3. Michael Salinas, Christof M. Jäger, Atefeh Y. Amin, Pavlo O. Dral, Timo Meyer-Friedrichsen, Andreas Hirsch, Timothy Clark, Marcus Halik, The Relationship between Threshold Voltage and Dipolar Character of Self-assembled Monolayers in Organic Thin-Film Transistors. J. Am. Chem. Soc. 2012, 134, 12648–12652. DOI: 10.1021/ja303807u.
2. Pavlo O. Dral, Timothy Clark, Semiempirical UNO–CAS and UNO–CI: Method and Applications in Nanoelectronics. J. Phys. Chem. A 2011, 115, 11303–11312. DOI: 10.1021/jp204939x.
1. Andrey A. Fokin, Tatyana S. Zhuk, Alexander E. Pashenko, Pavlo O. Dral, Pavel A. Gunchenko, Jeremy E. P. Dahl, Robert M. K. Carlson, Tatyana V. Koso, Michael Serafin, Peter R. Schreiner, Oxygen-Doped Nanodiamonds: Synthesis and Functionalizations. Org. Lett. 2009, 11, 3068–3071. DOI: 10.1021/ol901089h.
Photophysics of Organic Molecules
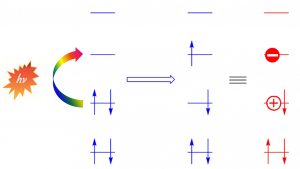
One of the most important steps in photovoltaic devices is photoinduced electron transfer (PIET).
Such materials as derivatives of fullerenes, porphyrins and (doped) polycyclic aromatic hydrocarbons (PAHs) can be used in organic solar cells and to mimic natural photosynthesis. We have used our own (semiempirical UNO–CI) and DFT methods to explain experimentally observed phenomena.
Our calculations elucidated the nature of the charge transfer band observed in UV-vis absorption spectra in donor-acceptor conjugates built of fullerene C60 as electron acceptor and free base and zinc porphyrin as electron donor[1] and in dumbbell-type donor-acceptor conjugates built of C60 (acceptor) and benzodifurane (donor).[3]
We have also suggested new candidates for PIET based on doped PAHs and C60 and porphin and predicted interesting properties of this complexes, e.g. change of direction of charge transfer upon change of environment.[2]
Publications
4. Pavlo O. Dral, Theoretical study of electronic properties of carbon allotropes. [online] Friedrich-Alexander-Universität Erlangen-Nürnberg, Dissertation (Dr. rer. nat.), 2013, http://opus4.kobv.de/opus4-fau/frontdoor/index/index/docId/3763. [cited 2 February 2014]
3. Hui Li, Christina Schubert, Pavlo O. Dral, Rubén Costa, Andrea La Rosa, Jürg Thüring, Shi-Xia Liu, Chenyi Yi, Salvatorre Filippone, Nazario Martin, Silvio Decurtins, Timothy Clark, Dirk M. Guldi, Probing Charge Transfer in Benzodifuran–C60 Dumbbell-Type Electron Donor–Acceptor Conjugates: Ground- and Excited-State Assays. ChemPhysChem 2013, 14, 2910–2919. DOI: 10.1002/cphc.201300378.
Appeared as an issue cover in ChemPhysChem: Hui Li, Christina Schubert, Pavlo O. Dral, Rubén Costa, Andrea La Rosa, Jürg Thüring, Shi-Xia Liu, Chenyi Yi, Salvatorre Filippone, Nazario Martin, Silvio Decurtins, Timothy Clark, Dirk M. Guldi, Inside Cover: Probing Charge Transfer in Benzodifuran–C60 Dumbbell-Type Electron Donor–Acceptor Conjugates: Ground- and Excited-State Assays (ChemPhysChem 13/2013). ChemPhysChem 2013, 14, 2870. DOI: 10.1002/cphc.201390062
2. Pavlo O. Dral, Milan Kivala, Timothy Clark, Doped Polycyclic Aromatic Hydrocarbons as Building Blocks for Nanoelectronics: A Theoretical Study. J. Org. Chem. 2013, 78, 1894–1902. DOI: 10.1021/jo3018395.
1. Alina Ciammaichella, Pavlo O. Dral, Timothy Clark, Pietro Tagliatesta, Michael Sekita, Dirk M. Guldi, A π-Stacked Porphyrin–Fullerene Electron Donor–Acceptor Conjugate that Features a Surprising Frozen Geometry. Chem. Eur. J. 2012, 18, 14008–14016. DOI: 10.1002/chem.201202245.
Reaction Mechanisms and Energetics
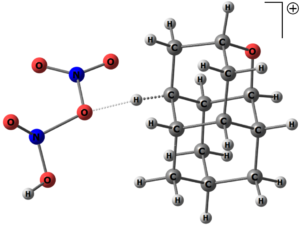
Functionalization of diamondoids influences properties of such devices, thus we have studied conditions for selective functionalization of oxadiamondoids and provided mechanistic explanation of experimentally observed products distribution, which can be used for future predictions.
We have shown that electrophilic nitration of oxadiamondoids proceed via H-coupled electron transfer (HCET) mechanism rather than via text-book 3c-2e transition states.[1] We have also demonstrated that this is true for other alkanes and with other nitration agents.[3]
In addition, we have shown that electron doping strongly influences relative stabilities of the dihydrogenated fullerene C60 and generally decreases energy differences between regioisomers.[2] We have also demonstrated how problematic is use of DFT methods for description of highly charged species with close relative energies.[3]
Publications
3. Pavlo O. Dral, Theoretical study of electronic properties of carbon allotropes. [online] Friedrich-Alexander-Universität Erlangen-Nürnberg, Dissertation (Dr. rer. nat.), 2013, http://opus4.kobv.de/opus4-fau/frontdoor/index/index/docId/3763. [cited 2 February 2014]
2. Pavlo O. Dral, Tatyana E. Shubina, Andreas Hirsch, Timothy Clark, Influence of Electron Doping on the Hydrogenation of Fullerene C60: A Theoretical Investigation. ChemPhysChem 2011, 12, 2581–2589. DOI: 10.1002/cphc.201100529.
1. Andrey A. Fokin, Tatyana S. Zhuk, Alexander E. Pashenko, Pavlo O. Dral, Pavel A. Gunchenko, Jeremy E. P. Dahl, Robert M. K. Carlson, Tatyana V. Koso, Michael Serafin, Peter R. Schreiner, Oxygen-Doped Nanodiamonds: Synthesis and Functionalizations. Org. Lett. 2009, 11, 3068–3071. DOI: 10.1021/ol901089h.