Methane Conversion via Photo-Driven Nitration
Activation of methane and its conversion to added-value products is an important topic which requires chemical solutions with high yields and selectivity. In our recent collaborative study, we present the oxidation of methane to methanol using nitrogen dioxide as a photo-mediator and molecular oxygen as the terminal oxidant. This process achieves the methane conversion with up to 17% while the selectivity towards CH3ONO2 is as high as 78%. Joined experimental and theoretical investigation support the HCl-catalyzed photo-nitration pathway.
The motivation behind the presented proposal is to address the challenges in selectively converting methane which is highly inert. Methane activation occurs naturally in the atmosphere through complex photoreactions involving NO2, but the synthetic potential of NO2 in large-scale methane conversion is not fully explored.
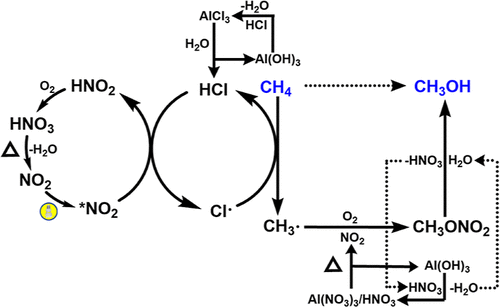
The plan is to construct a chemical looping for the photo-driven NO2-mediated CH4 conversion to CH3OH via CH3ONO2 as a gas intermediate. This strategy can prevent over-oxidation and obtain methanol as an important platform chemical through the hydrolysis of methyl nitrate.
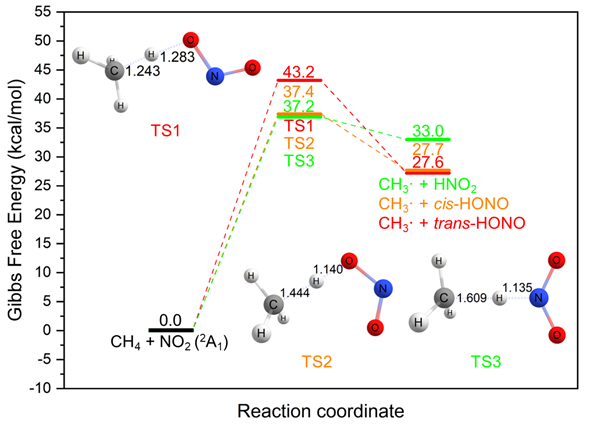
Excited NO2 has demonstrated the ability to activate alkanes, as evidenced by the successful photo-nitration of ethane at a low temperature of -10°C. However, the reaction between NO2 and CH4 does not take place until 180°C, at which temperature the methane is converted to COx as the main products. Theoretical investigations also support this experimental phenomenon. We found three different pathways for the reaction of CH4 with NO2 in the ground, 2A1 state (see figure below). Nevertheless, all of them have the Gibbs free energy barriers of 37-44 kcal/mol, which are too high for a reaction to take place at a low temperature of -10°C. We could not locate any reaction paths for CH4 and NO2 in the excited, 2B2 state.
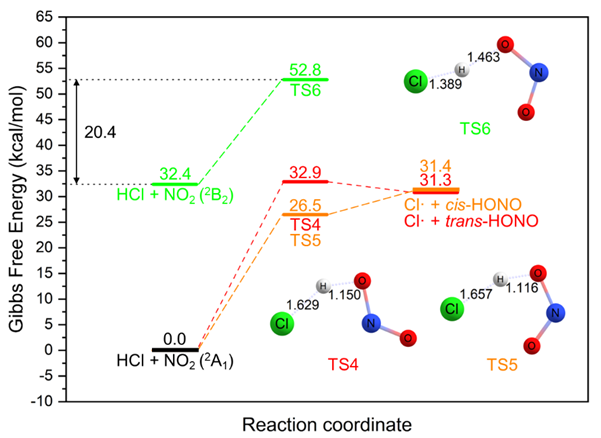
Fortunately, we found that HCl can catalyze methane photo-nitration via relay hydrogen atom-transfer (HAT) reactions. Theoretical investigations show that HCl can react with NO2 (2A1) in two different ways with lower free energy barriers (26–33 kcal/mol) than CH4 but the energy barriers are still not low enough to make these reactions happen easily at -10°C. After NO2 molecules are excited by the 450 nm LED illumination, HCl can react with NO2 (2B2) species with the low 20.4 kcal/mol free energy barrier (see figure below). Thus, the free Cl• radical can be readily generated from the HCl molecule via the H-abstraction by NO2 with the assistance of visible light. The existence of Cl• radical is supported by the online MS and a substantial amount of HONO is also detected after photoexciting a mixture of Al(NO3)/AlCl3.
The Cl• radical can then react with CH4 to regenerate HCl and produce CH3• radical. The HAT between CH4 and Cl• radical has a much lower barrier (about 1.67 kcal/mol) than that between CH4 and NO2 (37–44 kcal/mol). Then, CH3ONO2 can be selectively produced at a relatively low temperature via the reaction between CH3• radical and O2, NO2, NO. The produced CH3ONO2 can be hydrolyzed in water to produce CH3OH.
The concise representation of the catalytic loop shown at the top, summarizes the proposed mechanism. It starts with visible light exciting NO2 which is generated by heating aluminum nitrate Al(NO3)3. The excited NO2 then reacts with CH4 and O2, resulting in the production of methyl nitrate (CH3ONO2). This methyl nitrate is subsequently hydrolyzed to produce CH3OH. During the process, nitric acid (HNO3) and nitrate (NO3−) are generated and efficiently recycled back to Al(NO3)3. In the photo-nitration process of methane, HCl acts as a catalyst facilitating relay hydrogen atom transfer (HAT) reactions.
This work has opened up new opportunities in the field of alkane upgrading. The strategy proposed here can inspire the exploration of new pathways for the conversion and functionalization of other alkanes.
Publication:
- Xuefeng He, Lina Zhang, Jiawei Chen, Huichong Liu, Yuming Su, Han Li, Yonghua Cao, Pavlo O. Dral*, Cheng Wang*. Photo-Driven Aerobic Methane Nitration. Inorg. Chem. 2023, 62, 10343–10350. DOI: 10.1021/acs.inorgchem.3c01210.
Leave a Reply